Life on Another Planet?
Topic outline
-
Written by: Wilson Ho, EIS Education Team Member in collaboration with Miguel Rico and Suzanne Monir March 2016
Title of Lesson: Life on Other Planets
Topic: Evolution, Diversity of Species, Microbiological relevance in space
Grade (Age) Level: High School (Ages 14-18), UniversityThis course module is a brief summary of biological life on other planets. The topics covered include the difference between eukaryotic and prokaryotic cells, the conditions for a planet to be considered hospitable, the theories of how life began on Earth, and the hidden dangers of space travel.
Course Index:
- The Cell Theory
- Conditions for Life
- Origins of Life on Earth
- The Hidden Dangers of Space Travel
-
Forum
-
-
Since most cells are between 1 and 100 µm, they are invisible to the naked eye. Early microscopes in the 1600s opened up an entirely new field of biology: the study of cell biology and microorganisms. The idea that all living things are composed of cells developed over many years, and is strongly linked to the invention and refinement of this piece of technology.

Some of the first organisms to be studied were the single-celled planktonic organisms of pond water. These studies form the basis of cell theory, which encompasses the idea that cells are the fundamental units of life. All living things share a suite of characteristics: movement, respiration, sensitivity, growth, reproduction, excretion, and nutrition.The basic tenets of the cell theory are:
All living things are composed of cells and cell products.
New cells are formed only by the division of preexisting cells.
A cell contains the inherited information (genes) used as the instructions for growth, functioning, and development.
The cell is the functioning unit of life; all of the chemical reactions of life take place within cells.
Cells are separated into two main categories.
Eukaryotic cells
Prokaryotic cells
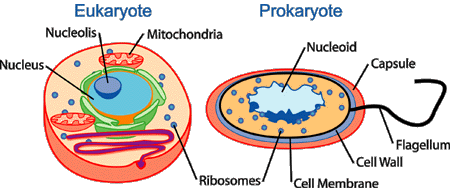
The word “prokaryote” translates in Greek to ’before nut’, which refers to the nucleus. They do not contain organelles or nuclei, are generally unicellular, and are much smaller than eukaryotic cells. Their genetic material is found a region called the nuclear area, but is not separated from the other cell material by a membrane. In terms of evolution, prokaryotes are believed to have formed well before eukaryotes - fossil records indicate that eukaryotes evolved from prokaryotes somewhere between 1.5 to 2 billion years ago. Eukaryotes are hypothesized to have formed when a prokaryotic cells was invaded by two smaller prokaryote cells in a process called symbiosis.
Eukaryotes are characterized by having their genetic material contained within a nuclear membrane, and are generally much larger than prokaryotic cells. The nucleus contains the DNA, while the nucleolus inside makes ribosomes. Eukaryotes are more complex cellular organisms and have a variety of internal membranes and structures called organelles (e.g. mitochondria, golgi apparatus, etc.). Fungi, protists, animals, and plants are all eukaryotic. -
At a grand 13.8 billion years old, the universe we live in still continues to expand. It is probable that we are not alone on the piece of rock we know as Earth; in the Milky Way, astronomers predict that there are 500 planet candidates that could support life. However, there are a few criteria that all planets must pass in order to be considered hospitable:
They must be within the “habitable zone”. The planet must be far enough away from the star it orbits due to the high heat and radiation, yet close enough to avoid extremely cold temperatures.
They must be made of a solid material. Planets like Jupiter and Saturn are not sustainable because they are made up of mostly hydrogen gas.
They must have a molten core. The liquid metal core in a planet gives rise to a magnetic field that protects from radiation during solar flares.
They must have an atmosphere. Without an atmosphere, essential gases for life like oxygen and carbon dioxide would dissipate immediately from the planet.
Simply put, the habitable zone is where water can remain in its liquid state without evaporating or freezing. One of the major factors in order to determine the location of this area in a solar system is the size of its star; a bright and large star would have a habitable zone very far from the centre. This makes it difficult for researchers to find candidate planets where life may exist, because every solar system is unique. We are lucky in that our Sun is just the right size; it is small enough to still have a long lifespan to develop complex life, and large enough to maintain a strong gravitational force to keep our planet in orbit. However our orbit around the sun is not the only factor, for our axial tilt is also very important. Some planets remain tidally locked to their star, which is similar to how the moon is tidally locked to the Earth. If the Earth was to be locked with our Sun, gravitational attraction would cause the same side of our planet to face the star. This would make both sides either too cold or too hot, essentially rendering Earth inhospitable.
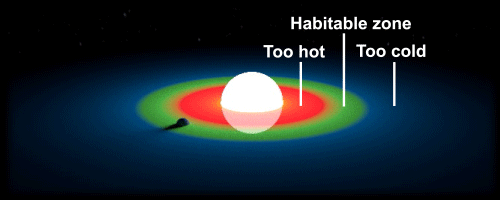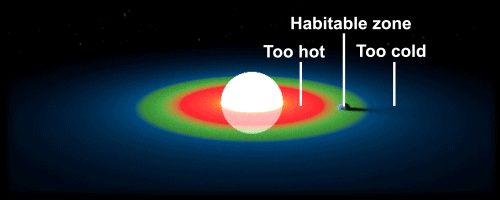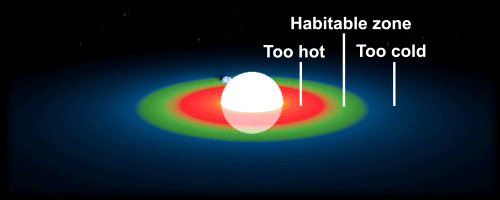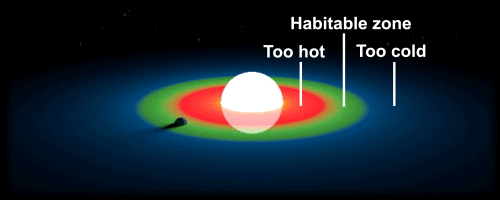
Without solid ground, life cannot exist. However, recent research has revealed that gas giants could transform into rocky planets as they age, making the planet eligible for life. Gas giants usually consist of a solid core surrounded by large amounts of hydrogen gas. The University of Washington has shown that it is possible for movements of gas (eg. the Coriolis Effect) to cause planets to migrate closer to the sun, and bring planets within the habitable zone. The planet would be exposed to comparatively higher doses of X-ray and ultraviolet radiation once within the area, leading to rapid evaporation of hydrogen gas. This would leave behind a solid core, making it perfect for colonization.

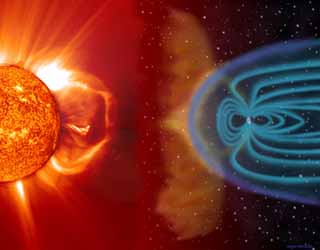
The main purpose of Earth’s iron core is to generate a magnetic field. Without it, Earth would be a volatile and inhospitable location due to the Sun’s radiation. Our planet is essentially one large magnet, with magnetic field lines extending thousands of kilometers into space. This field is generated using convection currents, which cause liquid metal at Earth’s core to constantly rise and fall. They defend against solar winds and coronal mass ejections (CMEs), which are bursts of dangerous radiation from the sun. Solar winds are streams of charged particles, known as plasma, that can reach over 800km per second at 800,000 ºC. CMEs are similar, but are released in short bursts instead of streams. Earth’s magnetosphere channels the radiation around the planet so we remain unharmed. If we were to be directly hit without our protective sphere, we would be stripped of our atmosphere, and all life would be quickly annihilated.
Among other things, Earth’s atmosphere is crucial in maintaining a relatively consistent temperature through a process known as the greenhouse effect. This is a system where greenhouse gases (mainly water vapour, methane, and carbon dioxide) trap solar radiation in the form of heat to return it back to Earth’s surface. These gases are special because of their molecular structure, which allows them to efficiently reflect infrared radiation. It is interesting to note that this is not the way real greenhouses work; they work by preventing nighttime winds from blowing away warm air trapped inside the room during the day. When radiation from the Sun hits the Earth’s surface, approximately 31% is reflected back to outer space, and 20% of the reflection is absorbed and reflected back by the atmosphere. This may not seem like a large percentage, but it is important to consider the monumental amount of energy coming from the sun. If we did not have an atmosphere, then our temperatures would be similar to the Moon; temperatures in the morning would easily reach 50ºC within a few hours. At night, temperatures would drop just as quickly, and our planet could reach sub-zero temperatures within a few hours. The idea behind anthropogenic global warming is that we, as a society, are enhancing the greenhouse effect by pumping tons of carbon dioxide into the atmosphere. This would lead to an increased amount of heat trapped within our atmosphere, which leads to the rise in global temperatures.

-
As humans, we constantly wonder about our evolutionary past. Where did we come from? How did we evolve? And fundamentally, what are the origins of life? Nobody knows for certain, but here are the 5 main theories for where life could have evolved on Earth. All these theories create environments that help build both amino acids for proteins, and nucleotides for genetic code. Once these components are made, RNA can be synthesized to perform basic metabolic processes. Evolution and time lead to more diverse and complex organisms, culminating in the 8.7 million different species we have today.
Ice provided a protective barrier to protect delicate compounds
Lightning provided the energy needed to spark life
Clay provides a framework to build and safeguard preliminary molecules
Deep sea vents catalyzed reactions in mineral rich environments
Panspermia: life was brought to Earth from outer space
Ice ages are frequent occurrences on Earth due to the high albedo rate of ice and snow, which are very good at reflecting the sun’s radiation back to space. Once the amount of ice and snow on Earth reaches critical mass, they trigger the Snowball effect to cause a positive feedback loop. Since more snow means more reflection of radiation, temperatures lower, causing even more snow to fall. It is a cycle that propagates itself to increase exponentially, culminating in an Ice Age. An experiment headed by Stanley Miller gives evidence that this theory is plausible by placing a solution of ammonia, which is theorized to have been plentiful on early Earth, in -42 degrees Celsius for 25 years; they found 7 different types of amino acids, and 11 types of nucleobases! We know that when exposed to low temperatures, chemical reactions should hypothetically slow down. However, while some reactions slow down, some actually speed up - this process is called eutectic freezing. As water freezes, very small pockets of water still remain in its liquid state. Impurities such as ammonia and cyanide do not freeze, and must stay within these microscopic pockets. This crowding makes it more likely for molecules to interact and bond together. Freezing also helps preserve the molecules once they form, since nucleobases would normally denature in a few days. Ice extends their lifetime to centuries, and gives molecules time to organize themselves into a structure that supports life.
Mary Shelley’s Frankenstein may not be as incredulous as we may think. A lightning bolt contains over 1 billion joules of energy, which is enough to power over 50 homes for a day. In the 1950s, Stanley Miller and Harold Urey tried to recreate the conditions of early life, and sent an electrical current through a solution of hydrogen, ammonia and methane (gases thought to be most common in Earth’s atmosphere). After a few days, the mixture turned brown, and they found a total of 23 different amino acids! The electric sparks provided the energy needed to break the bonds of molecules and recombine into a different configuration. However one of the main problems with this idea is that recent analyses have shown that the Earth’s atmosphere is actually composed of carbon dioxide, carbon monoxide, and nitrogen - no hydrogen. Therefore, this theory has been modified to include volcanoes! In the cloud of an erupting volcano, collisions between volcanic ash particles are able to maintain their charge to sustain the energy in a lightning bolt. Therefore, life could have began around volcanic islands, where the essential gases like hydrogen were present and plentiful. Yet another problem is the energy required. Although lightning could theoretically provide plenty of energy, bolts came in very short bursts. In Miller and Urey’s experiment, they had to provide the solution with a constant stream of electricity in order to have positive results. An interesting fact is that Miller did not actually fully complete his experiment. He never finished his follow-up, and packed the electrified solutions into a box. One of his grad students inherited the box, and finished his experiment 50 years later.

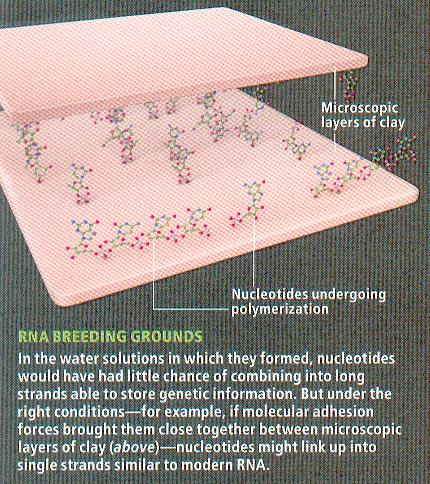
The main hypothesis for how clay could have been used to produce life is that it may have provided a framework for molecules to come together. Clay is made naturally in the ocean when silicates are leached from rocks. When immersed in water samples that are predicted to simulate ancient seawater, clay formed hydrogels under certain chemical and physical conditions (temperature, pressure, salinity, etc.). Hydrogels are linked polymers that can act as super sponges to soak up large quantities of water. An example is sodium polyacrylate, commonly found in babies diapers, which can absorb as much as 200 to 300 times its mass in water. Over billions of years, the compounds dissolved in the water that are trapped within the clay reacted to give rise to biological molecules. The clay acted as a barrier to protect the cells until they developed their own membrane, and provided a tight environment to increase the chances of interaction. In addition, researchers at Cornell found that DNA and RNA were both protected against nucleases and other destructive enzymes in clay, and transcription/translation reactions were greatly enhanced. They realized that biomolecules tend to adhere to clay’s surface, and that the cytoplasm of all cells behaves like a hydrogel structure. Finally, geological history found a correlation between the first appearance of clay, and the evolution of proto cells. The timeline proposed by both geologists and evolutionary biologists correlate nicely with this theory.
This theory originates in 1977, when scientists unexpectedly found life living around seafloor hydrothermal vents far from sunlight. Biologists have traced the most common ancestor of all life to aquatic microorganisms that lived in extremely high temperature environment, perfectly describing deep sea vents! These biological communities thrived on hydrogen, carbon dioxide, and sulfur: the necessary ingredients for microbial life. Many hypothesize that methanethiol, a sulfur and carbon compound, was the catalyst that helped create life. Scientists from the Woods Hole Oceanographic Institution dived deep under water to investigate this theory, and indeed found very high concentrations of methanediol. They are very excited, because this chemical could become a new indicator on other planets to see life exists. If rovers could land on Mars, or on any other celestial body, and find ice, they could drill through and measure methanediol concentrations to determine if life has, or still, exists! Another possible explanation is that vents provided the energy needed to bind molecules together to make more complex compounds. Evidence shows that billions of years ago, the oceans were filled with positively charged hydrogen ions, while deep-sea vents produced negatively charged hydroxide molecules. The vent walls created pores that separated the two from mixing, and created a natural charge gradient similar to a battery. The hydroxide molecules had to follow a specific path in order to reach the positively charged ocean, and this electric potential powered chemical transformations of carbon dioxide and hydrogen into amino acids, proteins, and genetic material.

Panspermia is one of the most popular and well researched theories, and it essentially states that life did not originate on Earth. The 3 main variations of this theory are lithopanspermia, ballistic panspermia, and directed panspermia. The first two ideas are very similar, and state that rocks expelled from another planet acted as a transfer vehicle to spread biological material. The main difference is distance; lithopanspermia is within the same solar system, while ballistic panspermia is between different solar systems. The third theory is the most controversial, and serves as a basis to a number of popular conspiracy theories and movies. Directed panspermia is the spread of genetic material by another more advanced extraterrestrial civilization. Acclaimed theoretical physicist and cosmologist Stephen Hawking subscribes to this theory, simply due to probability. The universe is unfathomably large, and it is almost statistically impossible for there not to be a planet similar to ours that could support life. He states that it is much more likely for life to come from outer space than it is for inorganic compounds to both combine together to form complex proteins, and simultaneously be able to sustain the rough conditions of primordial Earth. Strong telescopes, such as NASA’s Kepler telescope, are currently being used to identify candidate planets. However, due to the extensive distance between planets, it is hard to test our findings. The Search for Extraterrestrial Intelligence (SETI) Institute currently hypothesizes that alien life would be found within this lifetime. Extraterrestrial life might exist on a planet millions of light years away, or we might be alone in the vast universe; both statements are equally terrifying.

-
In 2016, the United States government gave 19.3 billion dollars to the National Aeronautics and Space Administration (NASA) to advance our understanding of space, provide space services, support the nation’s need for scientific knowledge. You may be wondering, why is space research so expensive? Why do we have to build advanced technologies to protect astronauts? Here are the 5 main reasons why you don’t want to go to space during your next Spring Break vacation!
Space debris
Radiation
Microgravity
Static Electricity
Dust
In 1978, American astrophysicist Donald J. Kessler published a paper highlighting the importance of space debris, and predicted that the density of trash in Earth’s orbit would be so great that random collisions would become inevitable. These random collisions would lead to even more debris, sparking a chain reaction that would quickly encircle our planet in a cloud of microscopic rubbish. Luckily, Kessler’s predictions have not yet come true, but the problem that he proposed still persists. Once a collision occurs in orbit, such as the 2009 crash between the Russian Cosmos 2251 and the American Iridium 33, large amounts of space debris is generated. That catastrophe alone created approximately 2000 pieces of trash larger than 10cm, and potentially hundreds of thousands of smaller fragments that cannot be tracked from Earth. These particles are immensely dangerous because of the low air resistance in space, making debris travel at astronomically high speeds. Fragments as small as 1 cm have the potential of destroying entire satellites, because of the amount of energy released in the collision. Remember that the formula for kinetic energy is a half times mass times velocity squared! Being hit by a sugar-cube in space would be the energy equivalent of standing next to a hand grenade on Earth! Currently, agencies around the world are monitoring space debris, and must maneuver satellites around large projectiles. Technologies are being developed to help cleanup the pollution that we have caused, for it is clear that something has to be done. Space in the universe is boundless, but the space around Earth is not.

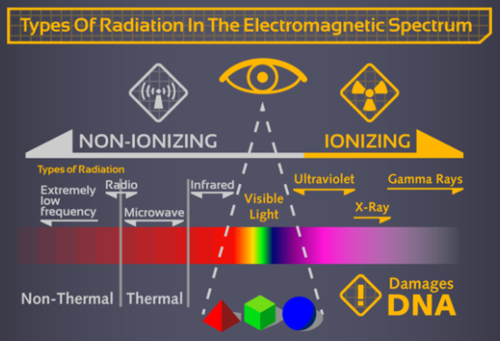
In a previous section you learned about solar flares and CME’s, and why Earth’s magnetic sphere is crucial for our protection. There are 2 main types of radiation: ionizing and nonionizing. Nonionizing radiation excites the matter it passes through, however it does not have enough energy to displace molecular bonds, or remove electrons from atoms. This form of radiation is benign, and is commonly found in the form of infrared waves, microwaves, and radio waves. Ionizing radiation is much more dangerous because it can disrupt major cellular functions by breaking the atoms of DNA molecules. Once broken, the proteins that are coded by that fragment of DNA cannot be made, leading to many serious problems downstream. Or, instead of breaking DNA directly, radiation can disrupt the water molecules within cells. Once hit, water would decompose into charged hydrogen and hydroxyl ions, called free radicals. These charged particles are not dangerous by themselves, but they can recombine to form other hazardous molecular compounds such as hydrogen peroxide. This chemical is very toxic, and could lead to the destruction of cells. This is why hydrogen peroxide is commonly sold in pharmacies as an antiseptic. Everyday, we are exposed to some amount of radiation. In our bodies, we have trace amounts of radioactive elements such as potassium-40, carbon-14, and radium-226. Natural radiation also comes in the form of uranium and thorium in the ground. Yet while we experience a radiation dosage of approximately 3 milliseverts a year, this is nothing compared the 100 milliseverts an astronaut can be exposed to for just 6 months on the International Space Station (ISS)! Even though solar radiation may have worked for the members of the Fantastic Four, this may not be the safest way to obtain superhuman abilities.
Floating around in space may seem like fun, but prolonged exposure to microgravity has many detrimental effects on the human body. Astronauts must exercise for up to 2 to 3 hours a day just to retain adequate muscle mass and bone strength to do work on the ISS. Their bodies become weaker because they no longer have to counteract Earth’s natural gravitational force when doing basic human activities, like standing up! If astronauts did not exercise, they would not have the physical strength to respond to any emergency aboard the spacecraft, and would be extremely weak upon return to Earth. Although muscle mass can be built back with therapy, bone density loss is much more difficult to rehabilitate. One of the specific changes caused by microgravity is orthostatic intolerance. When you lie down and then stand up very quickly, you may suddenly feel lightheaded for a short period of time. This is because gravity pulls blood away from the head, and your brain does not have enough oxygen to function properly; in order to counteract this, your heart naturally increases blood pressure and volume. However in space, there is no gravity, and this function becomes obsolete. Therefore astronauts may feel dizzy and lightheaded for a few days upon reentry to Earth because the body needs to learn how to respond to gravity again. When astronauts exercise, they deliberately increase their heart rate to ensure that their bodies remember how to increase blood pressure. They use three different types of equipment everyday to train: a bicycle, a treadmill, and a resistance exercise device (RED). A RED looks like a weight-lifting machine, and has a number of cords attached to pulleys and weights. All these exercise devices have been modified to work in microgravity. Unless you are a big fan of working out, space may not be right for you!

Although rubbing your socks on the carpet might be an amusing pastime, this seemingly harmless activity causes real trouble for astronauts. Static electricity can be generated using friction between two objects. Everything is made up of atoms, and each atom carries a certain number of electrons. These electrons can transfer from one object to another when you rub them together, and this generates a charge. This process is called triboelectric charging, and electrons will accumulate on the more electrostatic object. We know that these electrons do not want to be together because of Coulomb’s Law, which states that like charges repel. In a humid environment, water itself can act as a conductor to take the electrons away in order to prevent a large charge buildup. However, surfaces like the moon are very dry. Static can build from Moon dust rubbing against each other, and the surface of the Moon becomes very charged. After walking around on the surface of the Moon, astronauts can generate miniature lightning sparks every time they touch a conductor! This is a problem because a momentary shock of electricity could short circuit electronics, which are inconveniently made of metal. If we want to colonize the Moon, this problem can be solved by connecting the walls of the habitat to a large sheet/mesh of aluminum buried underground. NASA solved the problem of static electricity on Mars by attaching a reverse-lightning rod onto the the Mars Rover. This cheap alternative collects static electricity generated from friction between the wheels and the ground, and dissipates it into the atmosphere. This cheap alternative is impossible to implement on the Moon, because it does not have an atmosphere. On the bright side, if you want to become Thor and summon miniature lightning bolts, all you have to do is walk on the Moon and touch a conductor!

The famous footprint pattern made by astronaut Edwin Aldrin may look like mud, but it is actually made of dust! Dust is everywhere around us, so how can it possibly be so dangerous for astronauts? Since there is no water on the Moon, dust has a consistency similar to flour. It is a very fine powder, and can therefore easily get into small crevices such as the creases and seams of a spacesuit. This would not be a problem, except that this dust is made up of tiny jagged grains! Scientists found that it has properties similar to freshly fractured quartz or silica, and is lethal to human lungs. Around 16,000 people died when mining quartz because of silicosis (inhalation of silica) between 1968 and 2002! Astronauts aboard Apollo 17, the last manned mission to the moon, know firsthand how dangerous this material can be. The dust was so thick that it clogged up the joints in their suits, and rendered the astronauts nearly immobile! However, it became an even bigger problem when Commander Eugene Cernan and Lunar Module Pilot Harrison Schmitt forgot to brush the dust off of their boots before entering the space capsule. They were stuck with these particles for the rest of their journey, and began noticing the consequences of their mistakes immediately. Schmitt contracted hay-fever symptoms, and remained congested for the remainder of the voyage. In addition, it was almost impossible to clean the dust. Wiping down cameras and helmet visors became a near impossible task, because static electricity allows the dust to cling to any surface it attaches to. Luckily, upon return to Earth, scientists were able to use magnets to remove the dust. They found that each dust grain was bound by small iron particles that gave the dust its electrostatic properties. Remember to always clean your shoes before entering your home!


