Chemical Bonds
Topic outline
-
Written by: Suzanne Monir, EIS Education Team Member, December 2015
Title of Lesson: Chemical Bonds
Topic: Intermolecular and Intramolecular bonds, Surface tension, Capillary action
Grade (Age) Level: High School (Ages 14-18), UniversityThis course module is a summary of various intramolecular and intermolecular bonds that exist in chemistry with applications to surface tension and capillary action. The impact of bonding should be considered in its own right or as an integral aspect of the majority of microgravity experiments.
Course Index:
-
Forum
-
-
The types of bonding incorporated in Intramolecular bonding:
- Ionic
- Covalent
- Metallic
A review of the forces present within molecules.
Recall that intermolecular forces control the physical properties of a substance, while intramolecular forces control its chemical properties. Such properties include bond energy (heat required to break molecule into individual atoms) and flammability.
Ionic Bonding
Ionic bonds are electrostatic forces (attraction or repulsion of particles or objects because of their electric charge) that arise from the transfer of electrons from one atom to another. This type of bond forms between a metal and a non-metal, where the metal donates electrons to the non-metal, and results in oppositely-charged ions.
Ionic compounds, such as NaCl, form structures called crystal lattices. These structures consist of a network of ionic bonds, where the ions keep the same orientation to one another and repeat at regular intervals. The chemical formula of an ionic compound is the simplest whole number ratio of cations to anions within the crystal lattice and represents one formula unit.
Covalent Bonding
Covalent bonds arise from the sharing of valence electrons between two atoms. This type of bond normally forms between two non-metals. It results in the formation of either molecular compounds or network solids (complex structures composed of a continuous network of covalent bonds).
Coordinate covalent bonds are a special type of covalent bond where one atom donates both electrons in a bond as opposed to both atoms donating one electron. There is no difference in nature between a coordinate covalent bond and an ordinary covalent bond.
Electronegativity
Electronegativity is a measure of an atom’s electron attracting ability for a bonding pair of electrons. On a periodic table, it increases from left to right (closer to filling an orbital) and decreases down a group (more electron shielding). The noble gases, which all have an electronegativity of 0, are exceptions to this trend because they already have full orbitals. Elements with a high electronegativity have a strong attraction for electrons, while those with low electronegativity have a weak attraction for electrons. Fluorine is the most electronegative element.
 The difference in electronegativity between two bonded atoms gives rise to polarity if the electrons are not shared equally between them and there is a large difference in electronegativity. The electron density would be closer to the more electronegative atom, causing partial charges to occur: the more electronegative atom would have a partial negative charge and the less electronegative atom the partial positive charge. Partial charges are expressed with δ+/-.
The difference in electronegativity between two bonded atoms gives rise to polarity if the electrons are not shared equally between them and there is a large difference in electronegativity. The electron density would be closer to the more electronegative atom, causing partial charges to occur: the more electronegative atom would have a partial negative charge and the less electronegative atom the partial positive charge. Partial charges are expressed with δ+/-.Difference in electronegativity
Bond character
>1.7
Ionic
0.4-1.7
Polar covalent
≤ 0.4
Non-polar covalent
Metallic BondingIf a molecular has polar bonds, it does not necessarily mean the molecule overall is polar as the forces may cancel each other out, depending on the molecule’s geometry. (eg. CCl4)
Metals have low ionization energies and low electronegativities; thus, they easily lose their electrons. Because of this, in a metal, the metal atoms are positive ions packed closely together while their electrons are delocalized. This means that the electrons are free to travel from ion to ion, and spend little time with one particular ion. As such, metals are capable of conducting heat and electricity (electrons move freely), are malleable and ductile (electrons act as a cushion), and are lustrous (electrons on surface reflect photons).
Go to this animation from virtual laboratory, to review intramolecular bonding with animations: Intramolecular Animation
-
Dipole-Dipole Attraction
Many molecules contain bonds that fall between the extremes of ionic and covalent bonds. The difference between the electronegativities of the atoms in these molecules is large enough that the electrons aren’t shared equally, and yet small enough that the electrons aren’t drawn exclusively to one of the atoms to form positive and negative ions. The bonds in these molecules are said to be polar, because they have positive and negative ends, or poles, and the molecules are often said to have a dipole moment.
HCl molecules, for example, have a dipole moment because the hydrogen atom has a slight positive charge and the chlorine atom has a slight negative charge. Because of the force of attraction between oppositely charged particles, there is a small dipole-dipole force of attraction between adjacent HCl molecules.
Hydrogen Bonding
The hydrogen bond is a special case of dipole forces. A hydrogen bond is the attractive force between the hydrogen attached to an electronegative atom of one molecule and an electronegative atom of a different molecule. Usually the electronegative atom is oxygen, nitrogen, or fluorine. In other words – the hydrogen on one molecule attached to O or N that is attracted to an O or N of a different molecule. In other words, hydrogen bonds only occur when hydrogen is directly bonded to FON (fluorine, oxygen and nitrogen)
In the graphic below, the hydrogen is partially positive and attracted to the partially negative charge on the oxygen or nitrogen. Because oxygen has two lone pairs, two different hydrogen bonds can be made to each oxygen. This is a very specific bond as indicated. Some combinations that are not hydrogen bonds include: hydrogen to another hydrogen or hydrogen to a carbon.
Dipole-Induced Dipole
What would happen if we mixed HCl with argon, which has no dipole moment? The electrons on an argon atom are distributed homogeneously around the nucleus of the atom. But these electrons are in constant motion. When an argon atom comes close to a polar HCl molecule, the electrons can shift to one side of the nucleus to produce a very small dipole moment that lasts for only an instant.
By distorting the distribution of electrons around the argon atom, the polar HCl molecule induces a small dipole moment on this atom, which creates a weak dipole-induced dipole force of attraction between the HCl molecule and the Ar atom.
Induced-dipole induced-dipole (London Dispersion Forces)
As the electrons move about the nucleus, a momentary non-symmetrical electron distribution can develop that produces a temporary dipolar arrangement of charge. The formation of this temporary dipole can, in turn, affect the electron distribution of a neighboring atom. That is, this instantaneous dipole that occurs accidentally in a given atom can then induce a similar dipole in a neighbouring atom. This phenomenon leads to an interatomic attraction that is relatively weak and short-lived but that can be very significant especially for large atoms.
Now go through this animation to see how London Dispersion, Dipole and Hydrogen Bonding looks in an animation
Note, that London Dispersion forces = LD
Watch the following video by Oldsite Vanden Bout:
Or this more thorough explanation by Bozeman Science:
-
Recall that that there are three basic states of matter: solid, liquid, and gas.
In general, compounds with similar bonds have different physical properties depending on their mass. The heavier a compound is, the more London dispersion forces it has and thus it will be more like a solid. We compare how a solid will be a solid, liquid or gas by comparing their melting points (mp) or boiling points (bp). Melting point is the temperature that a substance is converted from a solid to a liquid, and the boiling point is the temperature that substance is converted from a liquid to a gas.
Have a look at this short animation to help you understand the difference.
The lighter a substance is, the lower the boiling point. For example, the boiling point of CH4 is much lower than SiH4 as the elements in the family get progressively heavier, they keep increasing.

BUT, look at the graph below.
 Some substances
clearly are not following this trend. In
particular, look at H2O compared to H2S. H2O clearly has a smaller mass
than H2S but H2O has the higher boiling point! Water is clearly an exception to the trends
we just discussed for London Dispersion.
And the reason is… there is much more going on than just London
Dispersion! What is the more predominate
intermolecular bond in H2O?
Well, we have hydrogen directly attached to oxygen so that can only be…
hydrogen bonds! Water is known as the
universal substance and is an important part of all biological beings. Water has a higher boiling point because of
its intermolecular forces!
Some substances
clearly are not following this trend. In
particular, look at H2O compared to H2S. H2O clearly has a smaller mass
than H2S but H2O has the higher boiling point! Water is clearly an exception to the trends
we just discussed for London Dispersion.
And the reason is… there is much more going on than just London
Dispersion! What is the more predominate
intermolecular bond in H2O?
Well, we have hydrogen directly attached to oxygen so that can only be…
hydrogen bonds! Water is known as the
universal substance and is an important part of all biological beings. Water has a higher boiling point because of
its intermolecular forces! Similarly, NH3 and HF, which also hydrogen bond, are anomalies in the trends of analogous compounds in their groups.
Quiz: 1Progress: 0 / 0 -
Quiz: 1Progress: 0 / 0
-
All biological compounds are impacted directly or indirectly by hydrogen bonds.
Proteins, which make up so much of our structures and enzymes, are full of hydrogen bonds.

Similarly, carbohydrates and nucleic acids also are dependent on hydrogen bonds in their 3-Dimensional structure.
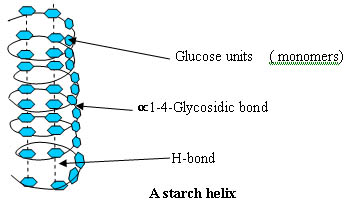
And Deoxyribonucleic acid (DNA):

Hydrogen bonds give us many of the coolest things in nature.
- Why water bugs can walk on water
- The reason geckos can walk on walls
- The reason water in plant vesicles seem to go AGAINST gravity in capillary action
- Why paper towels "soak" water
Surface Tension: (from Chemwiki)
Surface tension is the energy, or work, required to increase the surface area of a liquid due tointermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. water vs. gasoline) or solutes in the liquid (e.g. surfactants like detergent), each solution exhibits differing surface tension properties.
Introduction
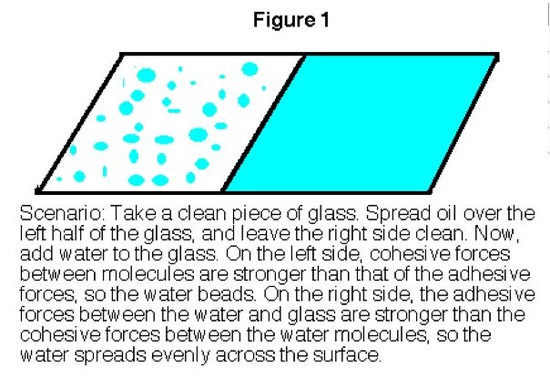
Whether you know it or not, you already have seen surface tension at work. Whenever you fill a glass of water too far, you may notice afterward that the level of the water in the glass is actually higher than the height of the glass (Figure 1 below). You may have also noticed that the water that you spilled has formed into pools that rise up off the counter. Both of these phenomena are due to surface tension.
How Does It Work?
In a sample of water, there are two types of molecules. Those that are on the outside, exterior, and those that are on the inside, interior. The interior molecules are attracted to all the molecules around them, while the exterior molecules are attracted to only the other surface molecules and to those below the surface. This makes it so that the energy state of the molecules on the interior is much lower than that of the molecules on the exterior. Because of this, the molecules try to maintain a minimum surface area, thus allowing more molecules to have a lower energy state. This is what creates what is referred to as surface tension. An illustration of this can be seen in figure 1 below.
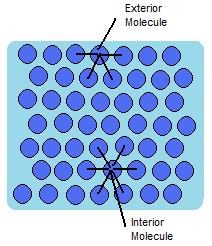
The water molecules attract one another due to the water's polar property. The hydrogen ends, which are positive in comparison to the negative ends of the oxygen cause water to "stick" together. This is why there is surface tension and takes a certain amount of energy to break these intermolecular bonds. Same goes for other liquids, even hydrophobic liquids such as oil. There are forces between the liquid such as Van der Waals forces that are responsible for the intermolecular forces found within the liquid. It will then take a certain amount of energy to break these forces, and the surface tension. Water is one liquid known to have a very high surface tension value and is difficult to overcome.
And as for those plants, that can be explained with Capillary action:
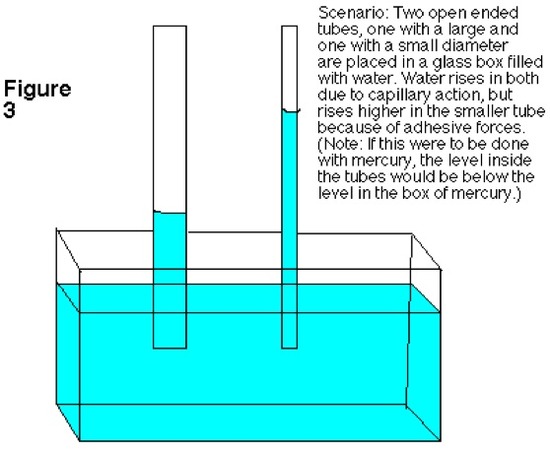
Capillary Action (from Chemwiki)
Scientific principles have the knack of explaining abnormal events that occur around us. A liquid, like water, ascending up a narrow tube naturally is not considered as a typical occurrence. Since, water and other liquids in general flows with the gravitational forces, it requires specific circumstances and physical effect to flow against the forces of gravity. This scientific phenomenon is called capillary action. The transportation of water from the roots to leaves of the tree happens due to the underlying principles of cohesion and transpiration.
Introduction
Capillary action can be defined as the ascension of liquids through slim tube, cylinder or permeable substance due to adhesive and cohesive forces interacting between the liquid and the surface. When intermolecular bonding of a liquid itself is substantially inferior to a substances’ surface it is interacting, capillarity occurs. Also, the diameter of the container as well as the gravitational forces will determine amount of liquid raised. While, water possesses this unique property, a liquid like mercury will not display the same attributes due to the fact that it has higher cohesive force than adhesive force.
Forces in Capillary Action
Three main variables that determine whether a liquid possesses capillary action are:
- Cohesive force: It is the intermolecular bonding of a substance where its mutual attractiveness forces them to maintain a certain shape of the liquid.
- Surface tension: This occurs as a result of like molecules, cohesive forces, banding together to form a somewhat impenetrable surface on the body of water. The surface tension is measured in Newton/meter.
- Adhesive force: When forces of attraction between unlike molecules occur, it is called adhesive forces.
Capillary action only occurs when the adhesive forces are stronger than the cohesive forces, which invariably becomes surface tension, in the liquid.
A good way to remember the difference between adhesive and cohesive forces is that with adhesive forces you add another set of molecules, the molecules of the surface, for the liquid to bond with. With cohesive forces, the molecules of the liquid will only cooperate with their own kind. Decreased surface tension also increases capillary action. This is because decreased surface tension means that the intermolecular forces are decreased, thus decreasing cohesive forces. As a result, capillary action will be even greater.

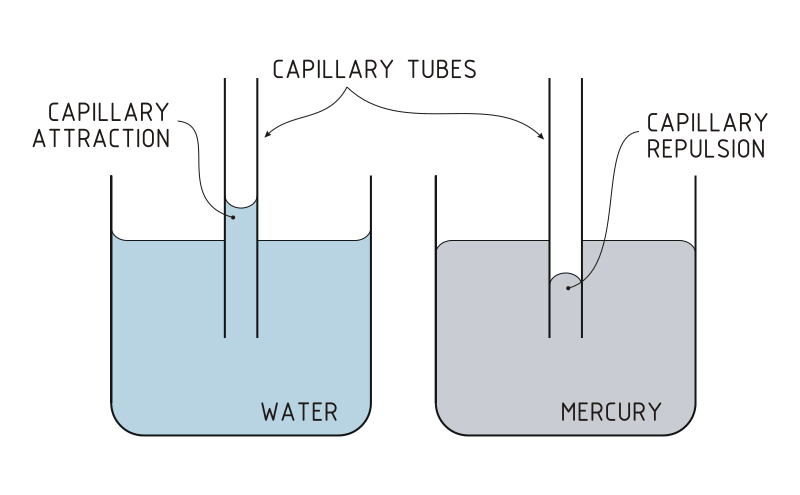
Figure 2:The scientific properties of surface tension and cohesion allow the water strider to calmly walk across water without drowning. http://commons.wikimedia.org/wiki/File:Water_Strider.jpg. Figure 4: It is possible to see that in water, the strength of the adhesive forces are larger than the strength of the cohesive forces. This results in the concave formation of water in the capillary tube; this is known as capillary attraction. Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away from the walls of the capillary tube. This is known as capillary Repulsion. http://commons.wikimedia.org/wiki/File:Capillary_Attraction_Repulsion_(PSF)(bjl).svg
So what happens with Surface Tension in Microgravity?
Watch this clip from Plasma Ben:
How this impacts your microgravity experiment design:
And now, as you design your experiments for microgravity, you must consider the differences in bonds. What will happen?
Here are some historical uses of chemical bonding in space exploration:
You may add more historical uses to the Feedback forum at the end of this course.
- ***
Here are some other links to articles relating to hydrogen bonding or surface tension in microgravity:
- Effect of microgravity on crystallization
- Condensation of supersaturated hydrogen bonding molecules
- NASA activity on surface tension
- The Dynamic behaviour of surface tension in microgravity

